A Novel Combination Therapy for Parkinson’s Disease
NU 2013-149
INVENTORS
- Dalton Surmeier Jr. (Northwestern University, Feinberg School of Medicine, Chair of the Department of Neuroscience)*
- Daniel Galtieri
- Ema Ilijic
- Enrico Zampese
- Jaime Guzman-Lucero
- Javier Sanchez
SHORT DESCRIPTION
This technology uses a low-dose combination of two FDA-approved compounds to reduce oxidative stress in neurons and slow disease progression in early stage Parkinson’s disease.
BACKGROUND
Parkinson’s disease affects millions and there are currently no disease-modifying therapies. Existing treatments mainly manage symptoms but often cause significant side effects. The high costs and limited efficacy of current options highlight the need for innovative approaches that address the underlying process of neurodegeneration.
ABSTRACT
This invention combines a dihydropyridine-based calcium channel inhibitor with a monoamine oxidase/nitric oxide synthase inhibitor in a low-dose formulation. The combination targets Cav1.3 channels and attenuates the consequences of nitric oxide signaling to lower oxidative stress, mitigating dopaminergic neuron loss in early stage Parkinson’s disease. In vivo laboratory studies validate the treatment's potential to slow disease progression effectively.
MARKET OPPORTUNITY
The global Parkinson's disease therapeutics market was valued at $6.59 billion in 2024 and is projected to reach $13.34 billion by 2034, growing at a compound annual growth rate (CAGR) of 7.39% (Source: Precedence Research, 2025). This growth is driven by the rising prevalence of the disease in the aging global population, with over 10 million people affected worldwide.. Driven by an aging population and the profound unmet need for disease-modifying treatments, this portfolio of selective Cav1.3 antagonists presents a strategic commercial opportunity.
DEVELOPMENT STAGE
TRL-3, Experimental Proof-of-Concept: Laboratory studies have validated the potential of this combination approach in reducing oxidative stress linked to Parkinson’s disease.
APPLICATIONS
- Disease-modifying therapy for Parkinson's disease: Slows disease progression by reducing oxidative stress.
ADVANTAGES
- Dual-pathway targeting: Combines two mechanisms to reduce oxidative stress effectively.
- Low-dose regimen: Minimizes risk of side effects while maintaining efficacy.
- Cost-effective development: Leverages off-patent drugs to lower overall expenses.
- Innovative treatment approach: Addresses an unmet need in modifying Parkinson’s disease progression.
IP STATUS
Issued US Patent 9,463,186
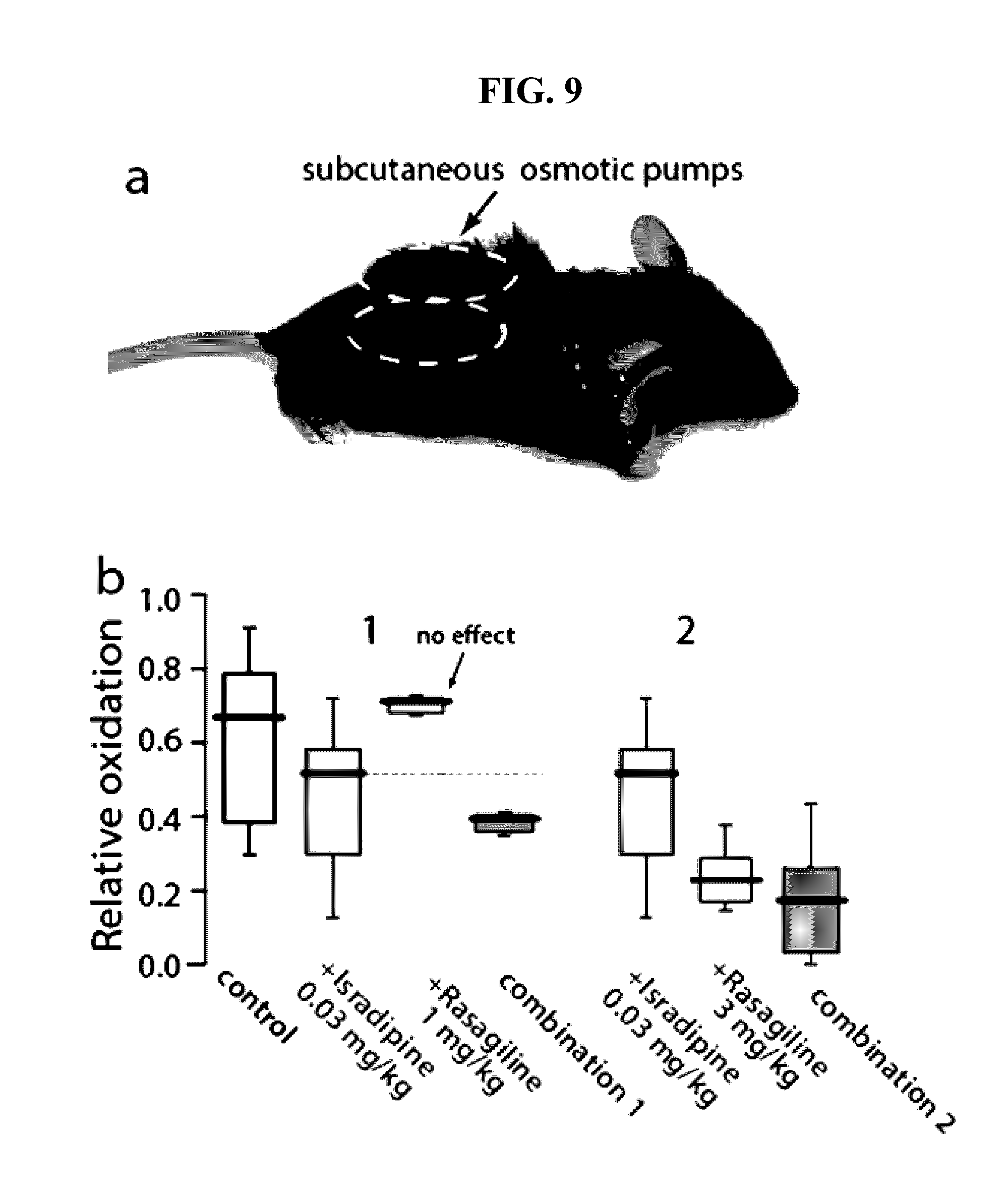
Combination of low dose isradipine and rasagiline is more effective than either alone. FIG. 9a —Mouse with two s.c. osmotic minipumps; drugs were given for 1 week prior to sacrifice. FIG. 9b —Box plots summarizing mitochondrial oxidant stress measurements in SNc DA neurons in ex vivo brain slices from mice given drugs alone or in combination. N>4 in each group.
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Categories:
Life Sciences > Therapeutics
Keywords:
Biomedical
Drug delivery
Neurodegenerative disease
Neurologic disease
PD - Parkinson's Disease
Therapeutics