The Use of Annexins in Preventing and Treating Muscle Membrane Injury
NU 2018-119
INVENTORS
SHORT DESCRIPTION
An annexin-based biological therapeutic that enhances the body's natural cell membrane repair process to reduce muscle cell death and preserve tissue function.
BACKGROUND
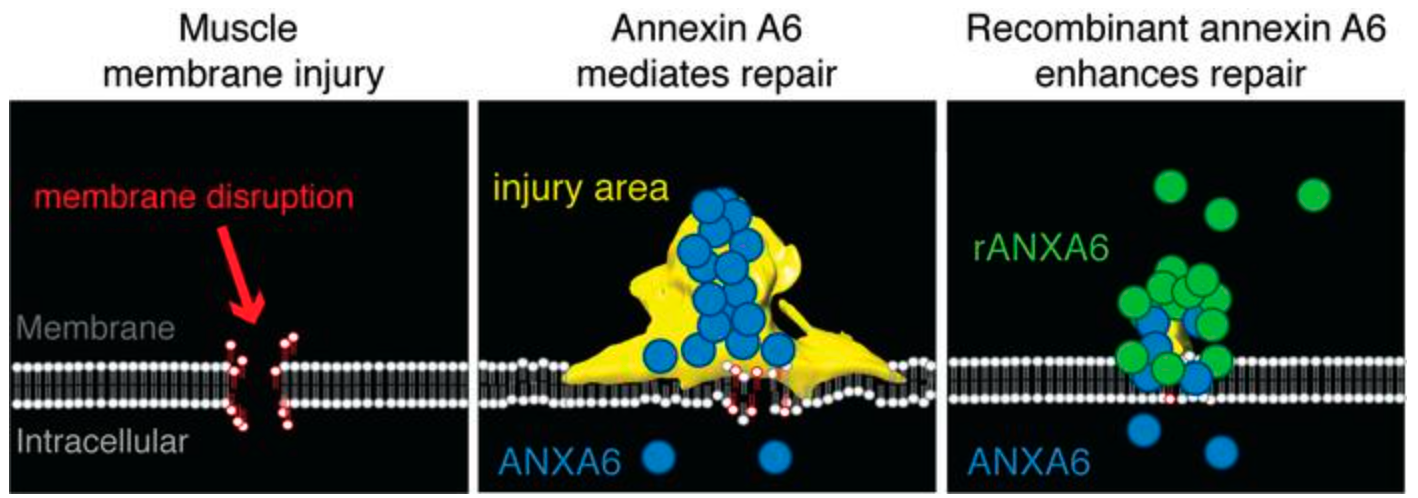 Cell membrane integrity is critical for survival, especially in tissues under constant mechanical stress like skeletal muscle. Devastating diseases such as Duchenne muscular dystrophy (DMD) are caused by fragile cell membranes that tear easily and repair slowly. Current treatments, primarily anti-inflammatory steroids, only slow disease progression and are associated with significant side effects. They do not address the fundamental defect in membrane stability and repair, creating an urgent unmet need for therapies that can protect against membrane damage, preserve muscle tissue, and improve patient outcomes.
Cell membrane integrity is critical for survival, especially in tissues under constant mechanical stress like skeletal muscle. Devastating diseases such as Duchenne muscular dystrophy (DMD) are caused by fragile cell membranes that tear easily and repair slowly. Current treatments, primarily anti-inflammatory steroids, only slow disease progression and are associated with significant side effects. They do not address the fundamental defect in membrane stability and repair, creating an urgent unmet need for therapies that can protect against membrane damage, preserve muscle tissue, and improve patient outcomes.
ABSTRACT
This technology provides compositions and methods for treatment of muscle cell cellular membrane injury. Annexins are essential for the calcium-dependent process of sealing membrane breaches. Administering a recombinant version of the annexin A6 protein directly bolsters the cell's endogenous repair machinery. Pre-clinical in vivo studies in mouse models of muscular dystrophy demonstrate that systemic administration of annexin A6 significantly reduces muscle fiber damage and lowers key serum biomarkers of injury, including creatine kinase. These results indicate that enhancing annexin activity is a promising therapeutic strategy for diseases characterized by cell membrane instability.
MARKET OPPORTUNITY
The global Duchenne muscular dystrophy (DMD) treatment market was valued at approximately $1.5 billion in 2023 and is projected to expand at a powerful CAGR of 13.5% from 2024 to 2030 (Source: Grand View Research, 2024).
DEVELOPMENT STAGE
TRL 4 - Prototype Validated in Lab: The therapeutic efficacy of recombinant annexin A6 protein has been demonstrated in vitro on isolated muscle fibers and in vivo in multiple mouse models of muscular dystrophy.
APPLICATIONS
- Therapeutic for Duchenne Muscular Dystrophy (DMD)
- Treatment for Limb-Girdle Muscular Dystrophies (LGMDs)
- Adjunct therapy for acute traumatic muscle injuries
- Cardioprotective agent following myocardial injury
ADVANTAGES
- Mutation-agnostic treatment for muscular dystrophies: Targets a common downstream pathology of membrane instability, making it applicable regardless of the specific underlying genetic mutation.
- Dual-action therapeutic benefit: Acts prophylactically to reduce susceptibility to membrane injury and actively enhances the repair of existing damage.
- Amenable to systemic administration: Can be delivered via injection to treat all affected muscles throughout the body, including the heart and diaphragm.
- Synergistic potential with other therapies: Can be used in combination with gene correction or anti-inflammatory treatments to provide a multi-pronged therapeutic approach.
PUBLICATIONS
IP STATUS
US Patent Pending 17/416,018. AU, BE, CA, CH, CN, DE, FR, GB, IE, JP, KR Patents Pending, and EP Patent Application Granted.
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Categories:
Life Sciences > Therapeutics
Keywords:
Neurodegenerative disease
Therapeutics