Focal Adhesion Proteins as Non-Invasive Diagnostics for Podocytopathies
SHORT DESCRIPTION
A diagnostic technology that detects focal adhesion complex proteins in bodily fluids for rapid and non-invasive identification of podocytopathies.
INVENTORS
- Lorenzo Gallon*, MD
- Robert Gerbasi
* Principal Investigator |
NU Tech ID: 2019-056
IP STATUS
US Patent Pending
DEVELOPMENT STAGE
TRL-3 - Experimental Proof-of-Concept Active R&D is initiated.
|
BACKGROUND
Current diagnostic methods for podocytopathies rely on invasive kidney biopsies and slow testing processes. Healthcare providers need a rapid, non-invasive method to accurately evaluate podocyte injury and kidney health.
ABSTRACT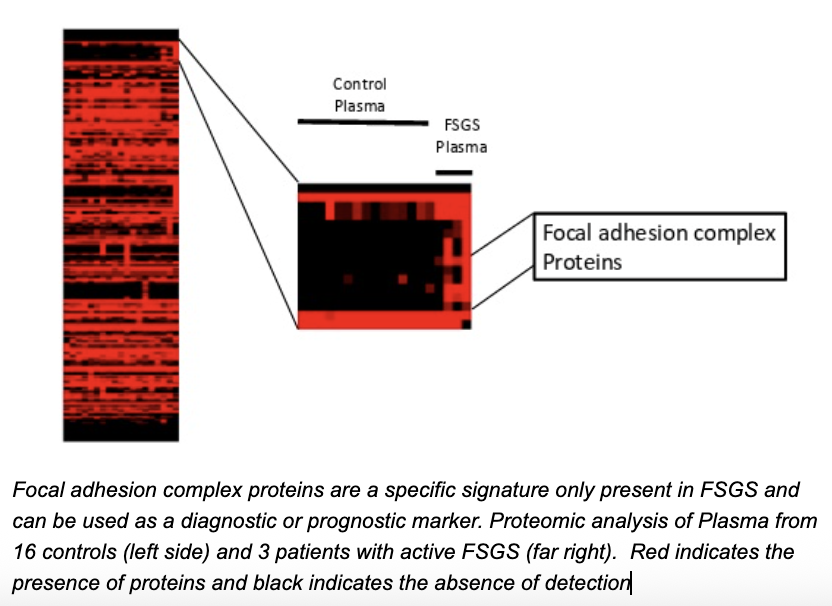
Northwestern researchers have identified several focal adhesion proteins present specifically in the plasma of patients experiencing podocyte effacement, a hallmark of various kidney diseases like minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS). These newly identified biomarkers offer a non-invasive alternative to kidney biopsies for diagnosing podocytopathies. Preliminary studies show promising sensitivity and specificity, providing timely insights into kidney health and patient management.
DEVELOPMENT STAGE
TRL-3 - Experimental Proof-of-Concept Active R&D is initiated: Key detection functions have been validated in preliminary laboratory experiments.
APPLICATIONS
- Diagnosis of FSGS and MCD: Enables non-invasive assessment of podocyte health.
- Prognostic indicator for treatment or transplant: Monitors protein levels to predict outcomes.
- Secondary patient screening: Identifies podocytopathies in patients with abnormal proteinuria.
- Drug toxicity evaluation: Assesses podocyte response during therapeutic interventions.
ADVANTAGES
- First non-invasive test for podocyte injury: Eliminates the need for invasive kidney biopsies.
- Faster diagnosis: Provides rapid results compared to conventional methods.
- Enhanced sensitivity: Detects subtle changes in podocyte protein levels.
- Improved patient monitoring: Facilitates timely adjustments in therapy through continuous tracking.
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Categories:
Life Sciences > Biomarkers & Biomedical Research Tools
Keywords:
Biomarker
Diagnostics