NU 2020-150
INVENTORS
- John Rogers (Northwestern University, Department of Materials Science and Engineering)*
- Igor Efimov (Northwestern University, Department of Biomedical Engineering, Medicine)*
- Roozbeh Ghaffari (Northwestern University, Department of Biomedical Engineering)*
- Mengdi Han
SHORT DESCRIPTION
This novel catheter platform delivers multimodal soft electronics for high-density mattping, increased contact with soft tissue and precise therapeutic interventions.
BACKGROUND 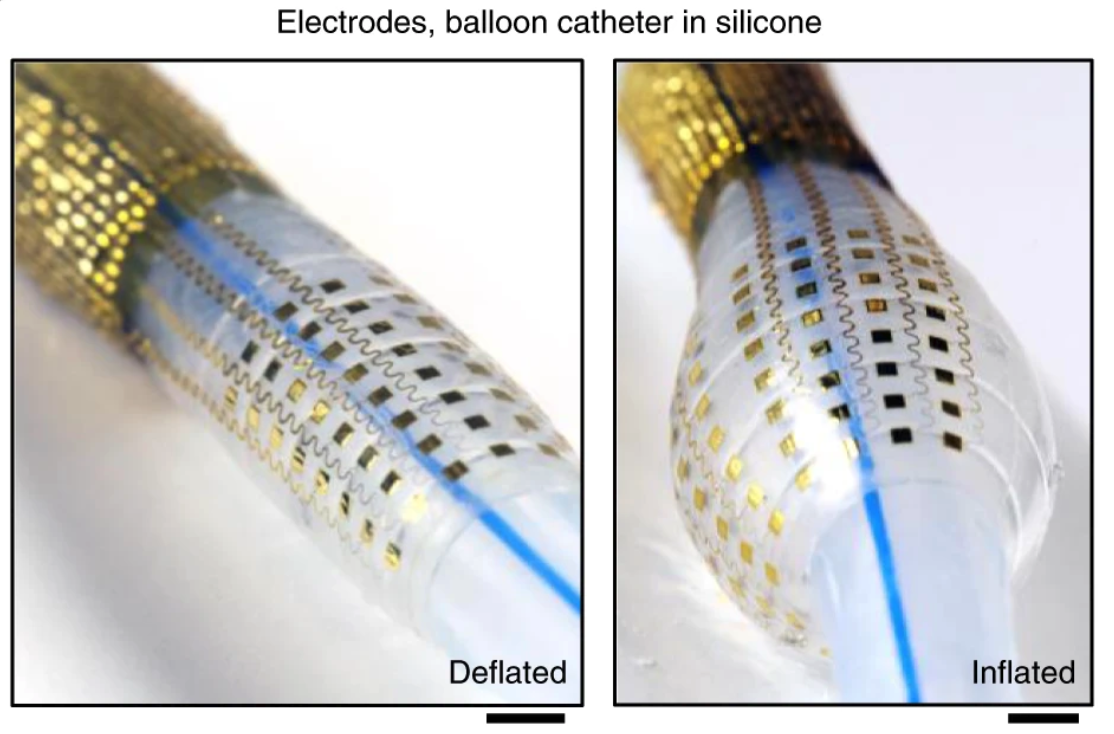
Current catheter-based procedures suffer from limited tissue interfacing and low sensor density. Existing devices require multiple catheters, increasing complexity, risk, and recovery times. There are unmet challenges stemming from the mechanical and resolution mismatch between current catheters and (cardiac) tissue.
ABSTRACT
The invention integrates soft multilayer electronics into advanced balloon catheter devices. The platform combines high-density sensor arrays with actuators that perform electroporation, radio frequency (RF) ablation, and drug delivery. Laboratory prototypes have validated effective tissue interfacing and multimodal operation, offering a streamlined solution for cardiac and other soft tissue interventions.
MARKET OPPORTUNITY
The global market for catheter-based procedures represents a massive and growing opportunity, with the cardiac ablation market valued at $5.15 billion in 2024 and the diagnostic catheter market at $4.39 billion (Sources: Towards Healthcare, 2025; Verified Market Research, 2024). These markets are projected to grow at strong CAGRs of 14.2% and 6.5% respectively, driven by the rising prevalence of chronic cardiovascular disease and a strong clinical preference for minimally invasive interventions.
DEVELOPMENT STAGE
TRL 4 - Prototype Validated in Lab: A laboratory-scale prototype has demonstrated the integration of high-density sensors and actuators for effective tissue interfacing.
APPLICATIONS
- Cardiac arrhythmia treatment: Electroporation and RF ablation procedures.
- Bladder and urethral tumor interventions: Sensing and ablation applications.
- Pulmonary therapeutic procedures.
- Gene therapy: Targeted drug and vector delivery via electroporation.
ADVANTAGES
- Improves tissue contact: Offers superior interfacing with soft tissues versus traditional catheters.
- Enhances sensor density: Provides high-resolution diagnostic data for precise treatment.
- Streamlines procedures: Integrates multiple functions into one device, reducing procedural complexity.
- Broad applicability: Supports a range of treatments from cardiac to oncological interventions.
PUBLICATIONS
IP STATUS
Issued US Patent US20230301595A1.