Catalytic Enantioselective Synthesis of 2-Aryl Chromenes
NU 2014-029
INVENTORS
- Karl Scheidt*
- Bi-Shun Zeng
SHORT DESCRIPTION
This invention outlines an innovative asymmetric catalytic method for synthesizing 2-aryl-chromenes with high yield and enantioselectivity. Utilizing palladium catalysis with chiral phosphoramidite ligands, the process provides a robust and scalable route to generate chromene derivatives that serve as versatile precursors for bioactive compounds.
BACKGROUND
 Chromenes are integral structural motifs found in numerous natural products and medicinal agents. Traditional synthetic approaches commonly deliver racemic mixtures and often require harsh conditions, limiting the substrate scope. This technology addresses these challenges by offering a refined catalytic system that minimizes side reactions, thereby enabling the efficient production of optically pure chromenes essential for pharmaceutical and agrochemical applications.
Chromenes are integral structural motifs found in numerous natural products and medicinal agents. Traditional synthetic approaches commonly deliver racemic mixtures and often require harsh conditions, limiting the substrate scope. This technology addresses these challenges by offering a refined catalytic system that minimizes side reactions, thereby enabling the efficient production of optically pure chromenes essential for pharmaceutical and agrochemical applications.
ABSTRACT
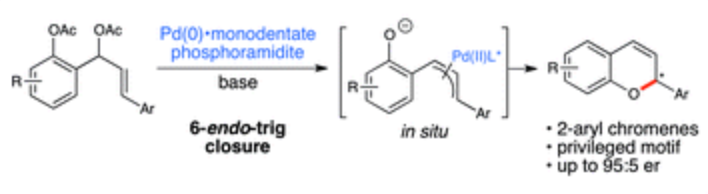
This invention presents a novel approach to the enantioselective synthesis of 2-aryl-2H-chromenes, achieving high yield and selectivity under mild conditions. The method uses o-arylallyl-substituted phenoxy ester starting materials in the presence of palladium(II) catalyst precursors and novel chiral phosphoramidite ligands. The method operates through base-promoted intramolecular cyclization via deacylation and C—O bond formation, enabling control of absolute stereochemistry. The process employs specially designed phosphoramidite ligands featuring piperidine-based or TADDOL-based scaffolds with sterically demanding aryl groups that interact with palladium to direct enantioselective allylic substitution, producing 2-aryl chromenes with enantiomeric ratios exceeding 90:10 and often reaching >95:5.
APPLICATIONS
- Pharmaceutical synthesis of biologically active chromene-based natural products and drug candidates.
- Synthesis of complex molecules containing the chromene framework, serving as intermediates for producing catechins, epicatechins, and other polyphenolic natural products.
- Enantioselective preparation of 2-aryl-2H-chromene scaffolds with various substitution patterns on both the chromene core and the aryl group, allowing access to diverse compound libraries.
ADVANTAGES
- High enantioselectivity with enantiomeric ratios typically exceeding 90:10 (often >95:5) across a wide range of substrates with varying electronic and steric properties, providing optical purities sufficient for pharmaceutical applications.
- Mild reaction conditions operating at room temperature with readily available starting materials derived from simple chalcones through two straightforward steps (reduction and acetylation), avoiding harsh reagents or extreme conditions.
- Broad substrate scope tolerating diverse functional groups including halogens, electron-withdrawing groups (nitro, trifluoromethyl), electron-donating groups (methyl, methoxy), and extended aromatic systems (naphthyl), demonstrating excellent functional group compatibility.
- Practical catalyst system using commercially available or easily synthesized palladium precursors with ligands that can be prepared through modular synthetic routes, allowing tuning of steric and electronic properties to optimize selectivity for different substrate classes.
PUBLICATIONS
IP STATUS
Issued US Patents:
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Categories:
Physical Sciences > Materials and Industrial Processes
Keywords:
Catalyst
Chemicals
Manufacturing/Processing
Materials