NU 2019-062
INVENTORS
- Karl Scheidt*
- Benjamin McDonald
- Richard Betori
SHORT DESCRIPTION
This technology provides a novel cooperative photoredox and Lewis acid catalytic approach to synthesize chromane scaffolds with high yield and stereoselectivity. It targets clinical development and natural product synthesis.
BACKGROUND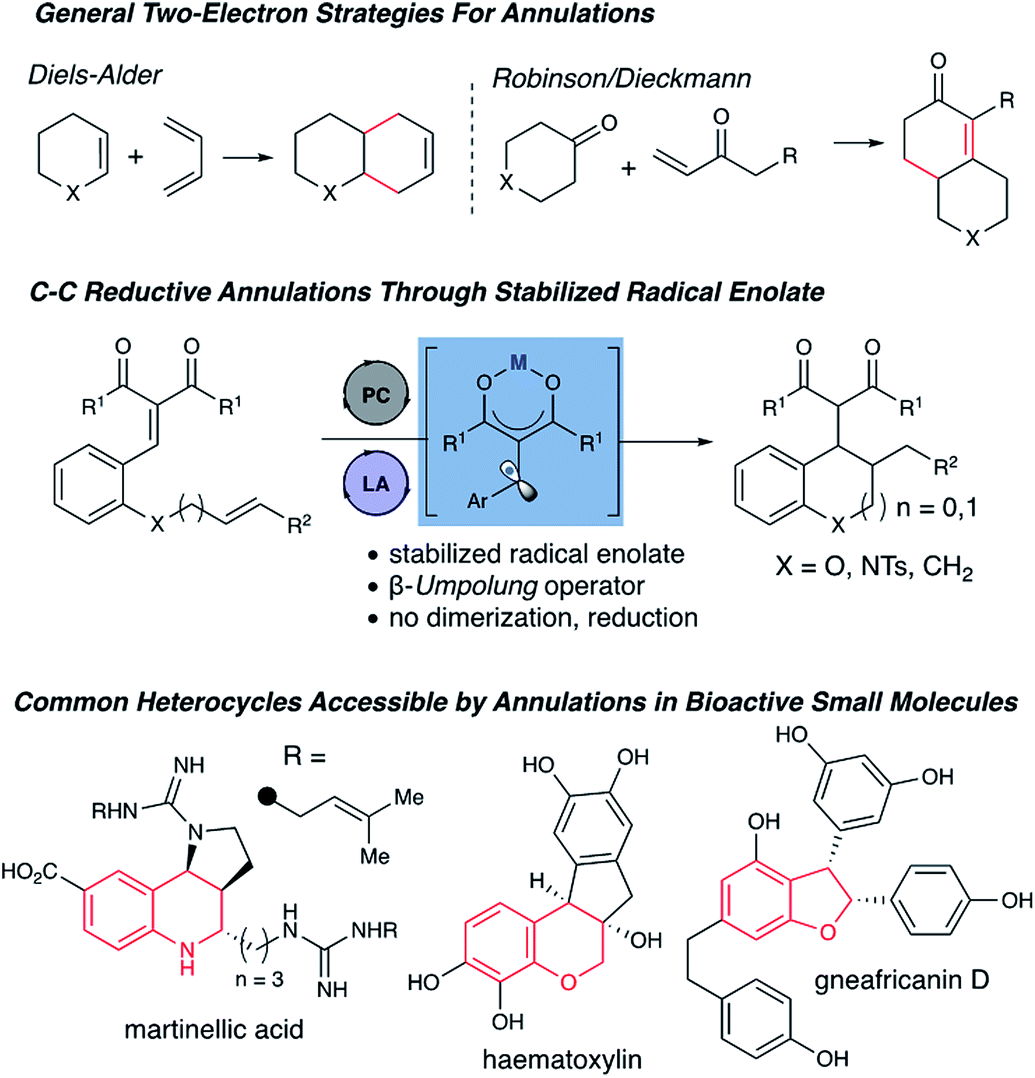
Chromanes are essential scaffolds found in many bioactive natural products and pharmaceuticals. Current synthesis methods often rely on toxic reagents and lack selectivity, creating a need for safer, efficient, and scalable production techniques.
ABSTRACT
The invention employs a cooperative catalytic system that couples photoredox and Lewis acid catalysis to achieve the reductive annulation of arylidene malonates with unsaturated electrophiles. Laboratory experiments demonstrated the formation of chromane products in up to 85% yield with favorable diastereoselectivity. The process leverages stabilized β-umpolung intermediates formed via a significant shift in reduction potential upon Lewis acid complexation, establishing a versatile platform for synthesizing densely functionalized heterocycles.
DEVELOPMENT STAGE
TRL-4 - Prototype Validated in Lab: Key functions have been demonstrated in a laboratory-scale prototype with consistent product formation and stereoselectivity.
APPLICATIONS
- Access to bioactive natural products: Synthesis of key chromane scaffolds for pharmaceutical use
- Clinical agent development: Production of novel compounds for targeted therapy
ADVANTAGES
- Facile access to pharmaceutically relevant scaffolds: Streamlines synthesis of complex molecules
- Diverse scaffold diversification: Enables new analog production beyond current methods
- High yield and selectivity: Achieves up to 85% yield with improved diastereoselectivity
- Avoids toxic reagents: Eliminates the need for stoichiometric tin hydrides
PUBLICATIONS
IP STATUS
Issued US patents 11,639,340 and 12,410,149